NEWS-Approval of moxidectin for a pilot onchocerciasis community treatment programme in Ghana
1 Jul 2025
News: Approval of moxidectin for a pilot onchocerciasis community treatment programme in Ghana
Authors: Annika Beate Wilder-Smith [1][2]
- Faculty of Medicine, University of Zurich, Zurich, Switzerland
- International League of Dermatological Societies
Conflict of interest: None
Key words: Moxidectin; onchocerciasis; community treatment; Ghana
In December 2024, Medicines Development for Global Health (MDGH) – a not-for-profit pharmaceutical company – announced that the Ghana Food and Drugs Authority had authorised the use of moxidectin 2mg oral tablets for the treatment of onchocerciasis in adults and children aged 4 years and older.1 This announcement declared Ghana the first onchocerciasis-endemic country to receive regulatory approval for moxidectin, marking an important step towards elimination goals and allowing the commencement of a pilot community treatment programme, the Momentum Project.
The Twifo Atti-Morkwa District in Ghana has been identified as a priority area for moxidectin use by the Ghana Health Service, after a higher-than-expected prevalence of onchocerciasis was detected despite multiple rounds of ivermectin mass drug administration (MDA). Following drug approval, the Momentum Project commenced in January 2025 after the arrival of the first drug shipment, which contained more than 250,000 moxidectin doses. Over the next three years, the Ghana Health Service will distribute moxidectin to the Twifo Atti-Morkwa community every 6 months, with the assistance of volunteers to promote engagement and door-to-door distribution to help eliminate parasite transmission and the presence of disease.
The launch of the Momentum Project is a significant step forward in efforts to eliminate river blindness and improve the lives of millions worldwide.
Dr John Lambert, General Manager of Atticus Medical
What is onchocerciasis?
Onchocerciasis, commonly known as ‘river blindness’, is a parasitic infection caused by the filarial worm Onchocerca volvulus. The parasite is transmitted by infected blackflies of the genus Simulium, which breed in fast-flowing, turbulent water.2 The blackfly ingests microfilariae when it bites an infected person; the microfilariae develop into larvae within the fly and then transmit to another human in subsequent bites.3 Larvae mature in the human host, and adult worms form nodules under the skin. The lifespan of the adult O. volvulus is approximately 10–15 years; therefore, exposed communities must be treated at least annually within this timeframe to eliminate transmission.
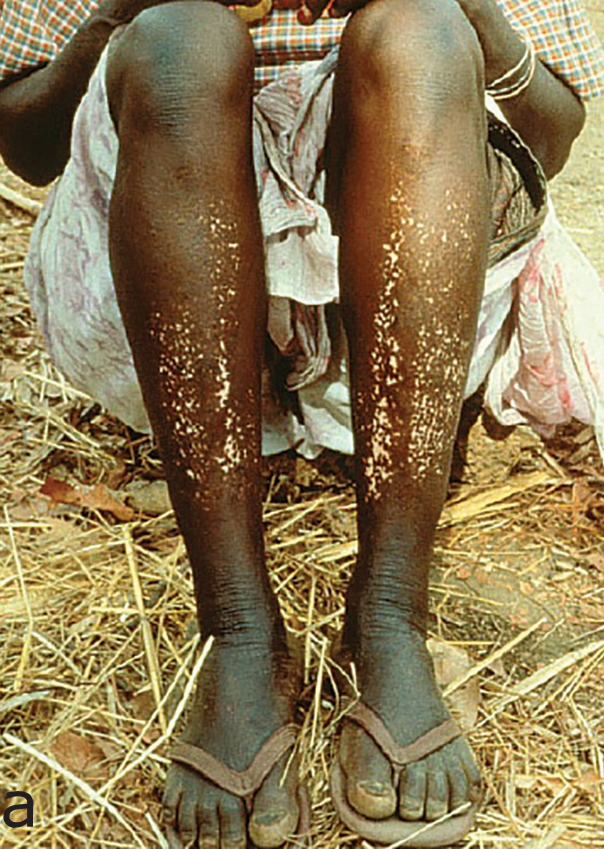
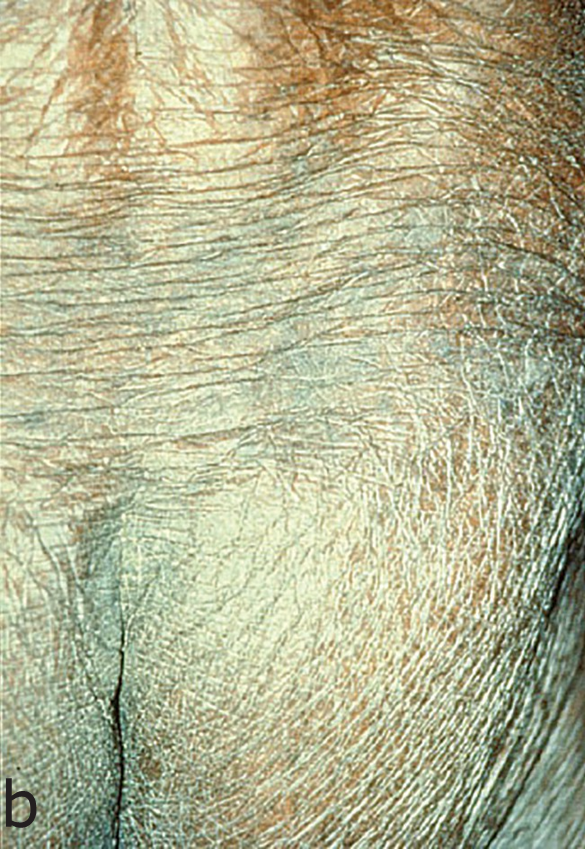
Onchocerciasis causes skin and eye pathology as microfilariae migrate within the subcutaneous tissue and induce intense inflammatory responses when they die. Onchocerciasis causes severe itching, disfiguring skin conditions (Figure 1) and visual impairment that can lead to permanent blindness.
Onchocerciasis is most common in sub-Saharan Africa but also occurs in the Republic of Yemen and Latin America.4 Currently, the strategy for disease elimination is via ivermectin MDA, requiring a minimum of 80% therapeutic coverage.3
In 2023, at least 249.5 million people required preventive treatment against onchocerciasis. In 2017, the Global Burden of Disease Study estimated that 14.6 million infected people already manifested skin disease, and 1.15 million suffered from vision loss.3 While ivermectin has significantly reduced the disease burden, new treatment options are needed to accelerate progress towards elimination.
What is moxidectin?
Moxidectin is a macrocyclic antihelminthic that binds selectively to parasite glutamate-gated chloride ion channels, required for nerve and muscle cell function.1 Ivermectin and moxidectin have microfilaricidal, embryostatic and irreversible female worm-sterilising effects.5 Adverse events are similar for both drugs when administered to patients with onchocerciasis. The common side effects include eosinophilia, pruritus, musculoskeletal pain, headache, lymphopenia, tachycardia, rash, abdominal pain, hypotension, pyrexia, leucocytosis, influenza-like illness, neutropenia, cough, lymph node pain, dizziness, diarrhoea, hyponatraemia and peripheral swelling.1 Phase II and III clinical trial data has demonstrated the superiority of a single dose of moxidectin (8mg) over a single dose of ivermectin (150 µg kg−1) in reducing and maintaining low skin microfilarial loads following treatment.5,6 Moxidectin is therefore expected to reduce parasite transmission between treatment rounds more effectively than ivermectin, thereby bringing forward the goal of elimination.
Past and present progress
Between 1974 and 2002, onchocerciasis was brought under control in West Africa with the help of the Onchocerciasis Control Programme, which involved the use of insecticides – released via helicopters and aeroplanes – against blackfly larvae and, since 1989, supplemental ivermectin MDA.3 In 1995, the African Program for Onchocerciasis Control (APOC) was launched with the aim of disease control in the remaining endemic countries, which transitioned to elimination by the end of 2015. The main strategy of APOC was to establish sustainable community-directed treatment with ivermectin combined, when possible, with environmentally safe vector control.3
The WHO roadmap for neglected tropical diseases 2021–2030 identified onchocerciasis as a target for disease elimination. The roadmap-set targets for onchocerciasis by 2030 are to eliminate the need for ivermectin MDA in at least one focus in 34 countries, to stop ivermectin MDA in more than 50% of the population in at least 16 countries and the entire endemic population in at least 12 countries.3 More than 172 million people were treated with ivermectin MDA in Africa in 2023,4 and in January that year, the Global Onchocerciasis Network for Elimination (GONE) was launched by the WHO, its member states and partners to accelerate progress towards meeting the WHO goals.3
In January 2025, Niger met the criteria for onchocerciasis elimination, marking the fifth country globally and the first country in Africa to interrupt transmission of O. volvulus.7 This success was achieved by vector control and MDA, which in this instance targeted lymphatic filariasis (LF) using ivermectin – which is effective against both LF and onchocerciasis – and albendazole. Since the target areas were also endemic for onchocerciasis, this intervention helped interrupt O. volvulus transmission. These interventions, combined with strong partnerships between the Nigerien government, the WHO and non-governmental organisations (NGOs), were pivotal in mobilising resources and wider support to achieve success. Currently, after decades of implementing successful elimination activities, five countries have been verified by the WHO as being onchocerciasis free: Colombia (2013), Ecuador (2014), Mexico (2015), Guatemala (2016) and Niger (2025).3
Where next?
Onchocerciasis elimination mapping has been developed to help countries conduct needs assessments and appropriate treatment rollout.3 This global mapping and modelling helps to identify where moxidectin MDA community treatment programmes would be most beneficial.
By identifying suitable target endemic regions and advancing strong collaborations between governments, the WHO and NGOs, other onchocerciasis-endemic countries can use Ghana’s approval of moxidectin for the treatment of onchocerciasis as an effective model for disease control. This announcement from Ghana represents a significant milestone in the initiative to eliminate onchocerciasis and enhance the lives of millions worldwide.
References
- Medicines Development for Global Health. 2024. Ghana becomes first river blindness-endemic country to approve moxidectin. Available at: https://www. medicinesdevelopment.com/news/ghana-becomes-first-river-blindness-endemic-country-to-approve-moxidectin (last accessed: 8 April 2025).
- Amazigo U, Leak S. Onchocerciasis and the African Programme for Onchocerciasis Control (APOC). Comm Skin Health J (Formerly J Comm Dermatol); 2008; 5: 13-5.
- World Health Organization. 2025. Onchocerciasis Factsheet. Available from: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis (last accessed 8 April 2025).
- World Health Organization. 2025. The Global Health Observatory. Onchocerciasis. Available at: https://www.who.int/data/gho/data/themes/topics/onchocerciasis (last accessed 8 April 2025).
- Kura K, Milton P, Hamley JID et al. Can mass drug administration of moxidectin accelerate onchocerciasis elimination in Africa? Philos Trans R Soc Lond B Biol Sci. 2023; 378:20220277.
- Opoku NO, Bakajika DK, Kanza EM et al. Single dose moxidectin versus ivermectin for Onchocerca volvulus infection in Ghana, Liberia, and the Democratic Republic of the Congo: a randomised, controlled, double-blind phase 3 trial. Lancet 2018; 392:1207–16.
- World Health Organization. 2025. WHO verifies Niger as the first country in the African Region to eliminate onchocerciasis. Available at: https://www.who.int/news/item/30- 01-2025-who-verifies-niger-as-the-first-country-in-the-african-region-to-eliminate-onchocerciasis (last accessed 8 April 2025).