Diagnostic clues of skin ulcers. Part II: ulcers of infectious aetiology
1 Jul 2025
Diagnostic clues of skin ulcers. Part II: ulcers of infectious aetiology
Authors: Cristina Galván-Casas
Dermatology Unit, University Hospital of Mostoles, Madrid, Spain.
Skin Neglected Tropical Diseases and Sexually Transmitted Infections Unit, Fight Infections Foundation, University Hospital Germans Trias i Pujol, Barcelona, Spain.
Abstract
This paper is the second of a two-part series of articles on clinical clues for diagnosing skin ulcers. The first part, which was dedicated to general concepts and ulcers with a non-infectious origin, was published in Community Skin Health 2024; 20(2):18-21. (https://www.ilds.org/what-we-do/our-foundation/csh-journal/).
In this second article, the most relevant types of ulcers with an infectious causative agent will be addressed, focusing on clinical clues to help identify the cause; the article also recommends confirmatory diagnostic tests and suggests suitable management and treatment strategies.
Conflict of interest: None
Key words: Skin ulcer; infectious skin ulcer; tropical ulcer; melioidosis; cutaneous diphtheria; yaws; Buruli ulcer; cutaneous leishmaniasis; mucocutaneous leishmaniasis; skin ulcer diagnosis; skin ulcer treatment
Key learning points
- A skin ulcer (lack of skin continuity) is a clinical sign with many different aetiologies.
- Clinical examination of the ulcer – and clues from other clinical and epidemiological findings – is essential for determining the cause and guiding subsequent diagnostic tests and treatment strategy.
- Ulcers tend to contain numerous micro-organisms, even when the primary cause is not infectious.
- For ulcers with no obvious diagnostic clues and when microbiological confirmation is not feasible, empirical treatment with cloxacillin or amoxicillin may be considered, and azithromycin or co-trimoxazole if there is no response.
Introduction
Regardless of the causative agent, the clinical features of many infectious ulcers are very similar.
The most frequent infectious agents can vary between regions and over time. Some studies have found Haemophilus ducreyi (Figure 1a), Streptococcus pyogenes and other facultative anaerobic bacteria, Streptococcus dysgalactiae, Arcanobacterium haemolyticum, and Corynebacterium diphtheriae among the major causes of nonspecific skin ulcers 1,2
When skin ulcers lack clinical clues indicating a specific causative agent, and microbiological confirmation is not feasible, empirical treatment should consider the local epidemiology. Cloxacillin or amoxicillin may be considered as first-line treatment , and azithromycin in a single dose of 30 mg/kg (maximum dose of 2 grams) or co-trimoxazole if there is no response.
Here, we will describe some infectious ulcers and give clues to facilitate their clinical diagnosis and treatment.
Tropical ulcer
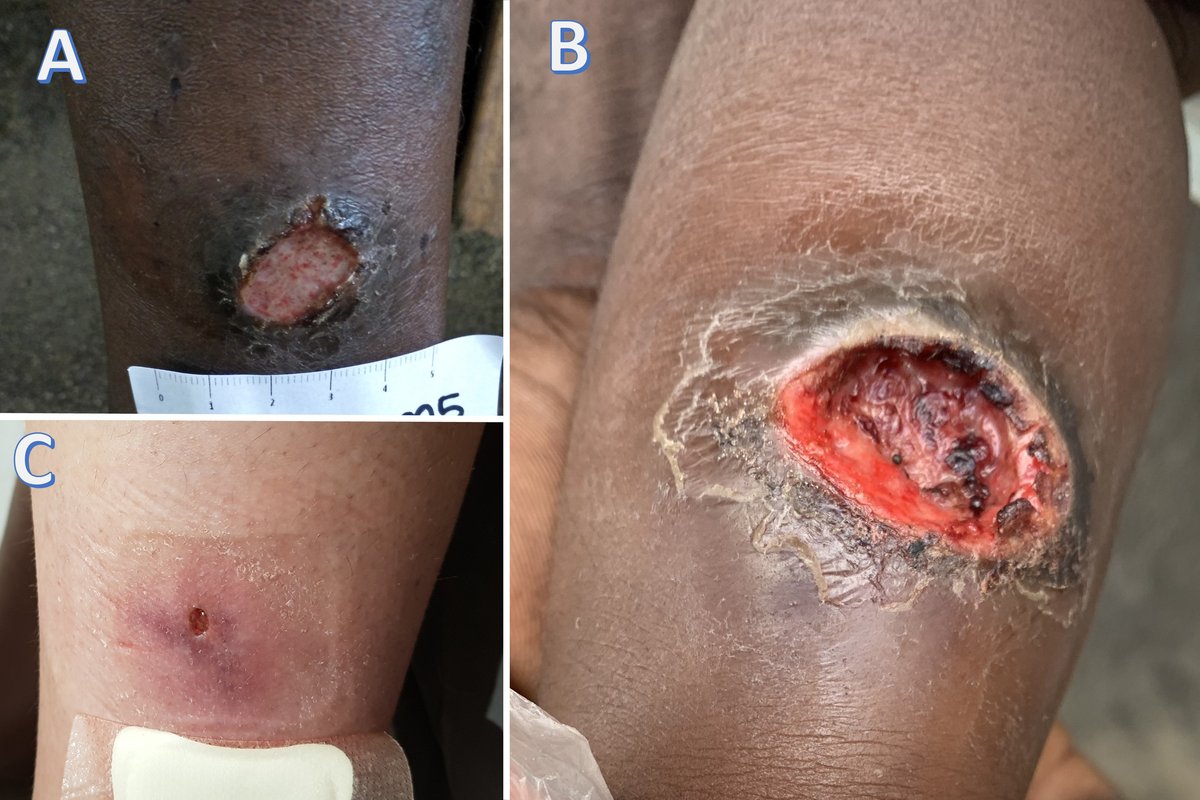
Tropical, or tropical phagedenic, ulcers are caused by polymicrobial skin infections that result in necrosis. Several strains of anaerobic bacteria, fusobacterium species, and spirochetes are often present. These ulcers are prevalent in warm, humid, and tropical rural regions, and mainly affect young adults and malnourished children in contact with mud or contaminated water. Chronic and debilitating diseases, such as malaria and soil-transmitted helminthiasis, are frequently found in association with these lesions. Trigger factors include skin injuries, such as trivial trauma, scratches, or insect bites.3
The ulcer tends to be round, single, painful, rapidly growing, and located on the lower leg. The initial lesion is a red papule or blister, surrounded by inflammation. The edges are well-defined, indurated, violaceous, and not raised. The ulcer bed is often necrotic, malodorous, exudative, and even purulent. Granulation tissue is often covered by a yellow membrane. The surrounding skin may be crusted and scaly (Figure 1b). Regional lymphadenopathy is usually absent (Table 1).
While the ulcer can heal within weeks, months, or years, leaving a residual scar without treatment, the infected lesion may lead to infection of deep structures, even affecting underlying bone.
Using Giemsa or Gram stains, direct microscopic examination of the swab from the ulcer bed and edges shows abundant fusobacterium species alongside gram-negative bacteria.
Treatment
- First-line treatment: metronidazole 30 mg/kg/day divided into 3 doses (maximum 500 mg per dose) for 10 days.
- Second-line treatment: tetracycline 500 mg every 6 hours for 1 week.
Cutaneous localized melioidosis
Melioidosis is a frequently underdiagnosed infection endemic in tropical and subtropical regions. The disorder is caused by Burkholderia pseudomallei, which is present in soil and superficial ground water. Previously described in Southeast Asia and Northern Australia, melioidosis is now considered an emerging disease following its detection in Africa and the Americas.
Systemic melioidosis is a severe opportunistic infection that is eventually fatal. Major risk factors include male sex, alcohol consumption, immunosuppression, and diabetes. Cutaneous melioidosis localized at the inoculation site is seldom fatal and usually affects children or young adults without risk factors. Commonly, cutaneous melioidosis usually presents as a single non-healing ulcer on the leg, surrounded by inflamed skin. Lesions can be multiple and appear elsewhere4 (Figure 1c, Table 1).
Diagnostic confirmation is difficult because identification tools are not widely available. B. pseudomallei is intrinsically resistant to penicillin and aminoglycoside.
Treatment
- First-line treatment: meropenem, imipenem or ceftazidime IV for 10–14 days followed by oral trimethoprim-sulfamethoxazole for 12–24 weeks.
- Second-line treatment: meropenem, imipenem or ceftazidime IV for 10–14 days followed by oral doxycycline or amoxicillin-clavulanate for 12–24 weeks.
In localised cutaneous melioidosis in young patients, in the absence of risk factors and proof of disseminated disease, oral antibiotics alone – trimethoprim-sulfamethoxazole, doxycycline or amoxicillin-clavulanate for 12–24 weeks – may be considered.
Cutaneous diphtheria
Cutaneous diphtheria is caused by Corynebacterium diphtheriae and C. ulcerans and is most prevalent in tropical and subtropical areas and in homeless people in any part of the world.5 C. ulcerans is a zoonosis transmitted from animals and contaminated raw milk; the reservoir of C. diphtheriae is humans. Bacterial entry points include minor abrasions, wounds, impetigo, eczema, scabies or burns, making clinical presentation highly variable.
Ecthyma diphthericum is the typical lesion, which is usually located on exposed areas. The lesion starts with a yellowish vesicle or pustule evolving into one or more chronic, non-healing, shallow ulcers, with marked raised or sunken edges, sometimes covered by a dirty grey crust (pseudomembrane). Untreated, healing time can vary from weeks to years.
Typical signs include a serosanguinous exudate on a brown-grey adherent pseudomembrane; while pain is present at the beginning, the lesion becomes painless during the following weeks (Table 1).
Regardless of entry point (e.g., respiratory or cutaneous), all C. diphtheriae and C. ulcerans can generate exotoxins, although cutaneous diphtheria only causes systemic complications (e.g., myocarditis, dysphonia, paralysis) if there is extensive infection.
Ulcers are frequently contaminated by other Gram-positive bacilli such as Streptococcus pyogenes and Staphylococcus aureus. This contamination – along with the abundance of other corynebacteria on the skin microbiome – makes confirmatory diagnosis very difficult. While C. diphtheriae or C. ulcerans may be cultured from a bacterial wound swab, laboratory processing for diphtheria may not be routine, necessitating the provision of complete clinical information to alert the laboratory to consider this culture.
Treatment:
- First-line treatment: erythromycin 40 mg/kg/day (maximum 2g/day) administered in divided doses (10–15mg/kg every 6 hours; maximum 500 mg per dose) for 14 days.
In severe or extensive cases, diphtheria antitoxin should be added.
Buruli ulcer
Caused by the acid-fast bacillus Mycobacterium ulcerans, Buruli ulcer is the third most common mycobacterial infection after tuberculosis and leprosy. Cases are detected in the tropics in West Africa, the Americas, Asia, and the Western Pacific. Deforestation and anti-ecological practices favour Buruli ulcer development.6
M. ulcerans is isolated from soil, vegetation, standing or slow-flowing water, and various animals. Being non-transmissible between humans, mosquitoes and other insects are the suspected transmission agents. In southeastern Australia, Buruli ulcer cases are increasing, and the same M. ulcerans genome has been detected in infected individuals, mosquitoes, and possum faeces within geographical correlates.7
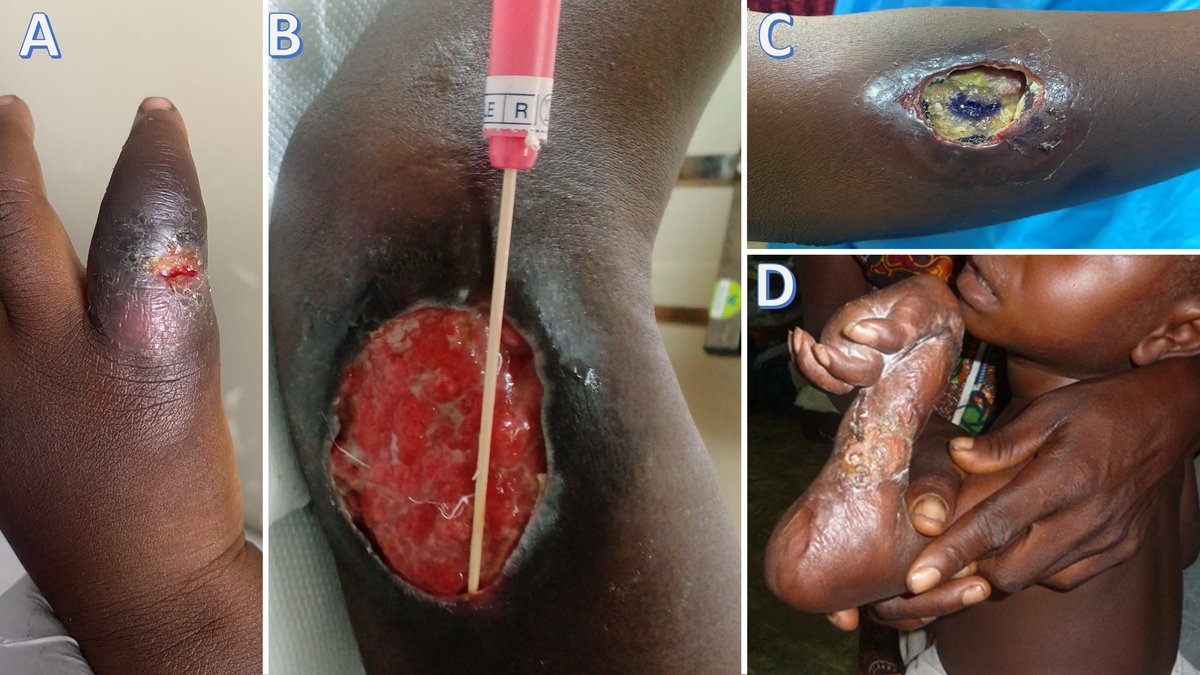
M. ulcerans secretes mycolactone – a cytotoxic and immunosuppressive exotoxin – which blocks pain receptors and rapidly diffuses into subcutaneous tissues, causing dramatic painless tissue destruction without any associated features of systemic inflammatory response (e.g., fever, malaise, lymphadenopathy) (Figure 2). Superinfection by other bacteria may cause pain and lymphadenopathy.
Buruli ulcers are more common in children under 15 and women (Table 1); HIV co-infection increases susceptibility and severe treatment-resistant forms. The ulcers mainly occur on the legs and arms, although the trunk and face may also be involved. The clinical course follows different phases and lesions can be classified in terms of severity (Table 2).
In most cases, the diagnosis is clinical. Laboratory culture is difficult and slow. Direct microscopy (Ziehl's staining) requires expertise and careful examination of biopsy material; biopsy sensitivity is variable but higher in recent lesions.
Polymerase Chain Reaction (PCR) fine needle aspiration or swabs from the undermining ulcer edges (Figure 2b) are more useful than biopsy. There is a network of laboratories supporting disease control programmes offering this technique. Other point-of-care tests, such as loop-mediated isothermal amplification (LAMP), facilitate early diagnosis and are a research priority.
Treatment:
Buruli ulcer requires prompt recognition and treatment to prevent deformities and disabilities (Fig. 2D). Treatment includes different approaches.
Antibiotics
- First-line treatment: rifampicin 10 mg/kg daily for 8 weeks, plus clarithromycin 7.5 mg/kg twice daily for 8 weeks.
- Second-line treatment (recommended only for adults): rifampicin 10 mg/kg daily for 8 weeks plus moxifloxacin 400 mg daily for 8 weeks.
New shorter-duration regimens and new drugs effective against mycobacteria, e.g., telacebec, are under study.
Other measures
- In endemic locations, wound care and monitoring can help with prevention of unwanted sequelae;
- Bacillus Calmette–Guérin (BCG) vaccination provides some short-term immunity and protection against osteomyelitis;
- Local heat application (above 37°C) can be of use (M. ulcerans is thermosensitive);
- Surgical debridement of necrotic areas and reconstruction of defects by grafts or flaps may be needed in selected cases;
- Physiotherapy and rehabilitation can help prevent and treat contractures.
A paradoxical reaction – worsening clinical signs caused by increased host immunity – can occur 3–10 weeks after antibiotic initiation. In this situation, culture and PCR monitoring are required to rule out bacterial superinfection, which may be confused with paradoxical reaction. Bacterial superinfection should be treated according to microbiological results.
Yaws ulcer
Yaws is a treponematosis caused by Treponema pallidum pertenue that is not sexually or vertically transmitted. Associated with poverty, yaws is found in some warm, humid regions between the tropics of Cancer and Capricorn in Africa, Asia, Central and South America and the Pacific Islands.
The disease usually affects the legs, feet, and buttocks of children under 15 (Table 1).
Transmission occurs by direct contact with a skin lesion or its secretions. The spirochete cannot cross an intact skin barrier and requires a break in the skin (e.g., a wound or scratch) to colonise.8
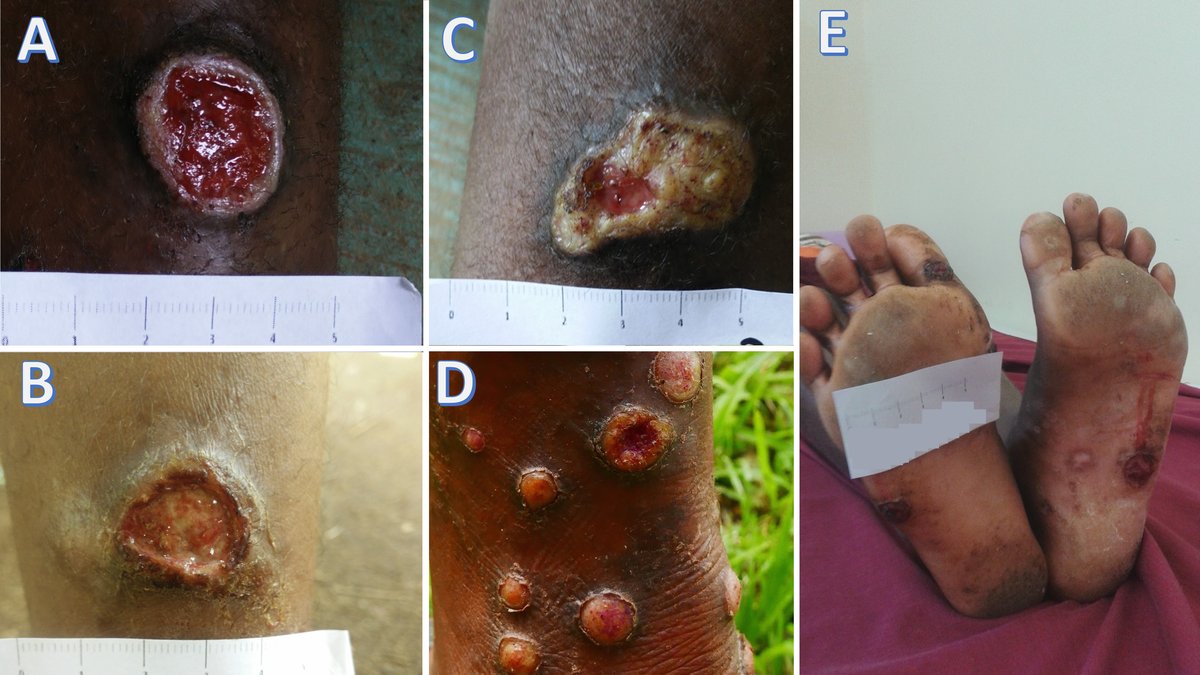
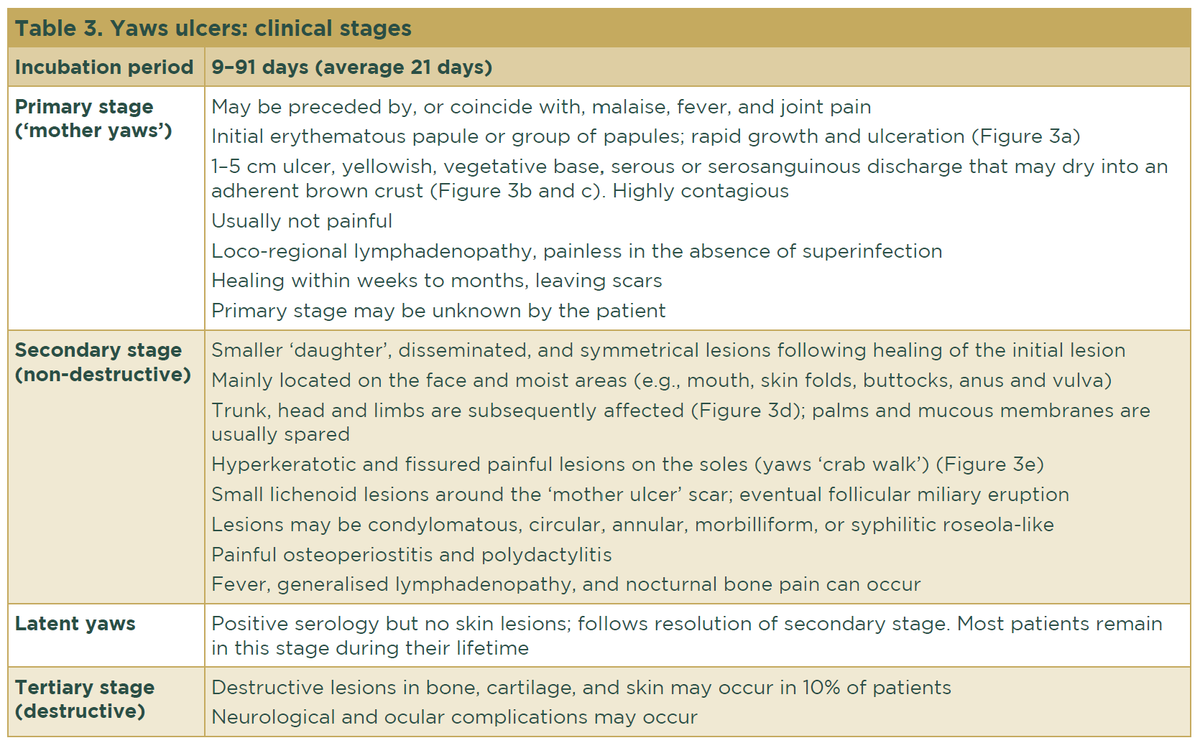
Similar to syphilis, the clinical course of the disease follows different stages (Table 3), and its diverse and nonspecific clinical manifestations make diagnosis complicated. Primary yaws is confused with other tropical skin ulcers, while secondary lesions are confused with insect bites, chronic scabies, or verrucae.
Expertise is required for dark-field microscopic examination of an ulcer swab or discharge fluid to confirm the presence of treponemes. The Dual Path Platform (DPP) is a useful, rapid serological point-of-care test that detects treponemal antigens (indicating past posititivity, latent or active infection) and nontreponemal antigens (positive in active infection). Molecular tests, although more sensitive, are less available.
Treatment
- First-line treatment: single oral dose of azithromycin 30mg/kg (maximum 2 grams).
- Second-line treatment: benzathine penicillin IM 1.2 MU in patients over ten years old and 0.6 MU in children under ten years.
Leishmania ulcer
More than 20 Leishmania species cause this global parasitic infection, which is most prevalent in the Americas, Mediterranean Basin, Middle East, and central Asia. The parasite is transmitted to mammals by the bite of different species of blood-sucking female phlebotomine sandflies. Approximately 70 animals, including humans, are the source of infection.
The development and severity of the disease are associated with malnutrition, unsuppressed HIV infection or other causes of immunosuppression, population displacement, deforestation, and poor housing. There are three forms of leishmaniasis: visceral (VL), cutaneous (CL), and mucocutaneous leishmaniasis (MCL). Visceral leishmaniasis (kala-azar) is a severe disease – fatal if untreated – which will not be addressed in this text.
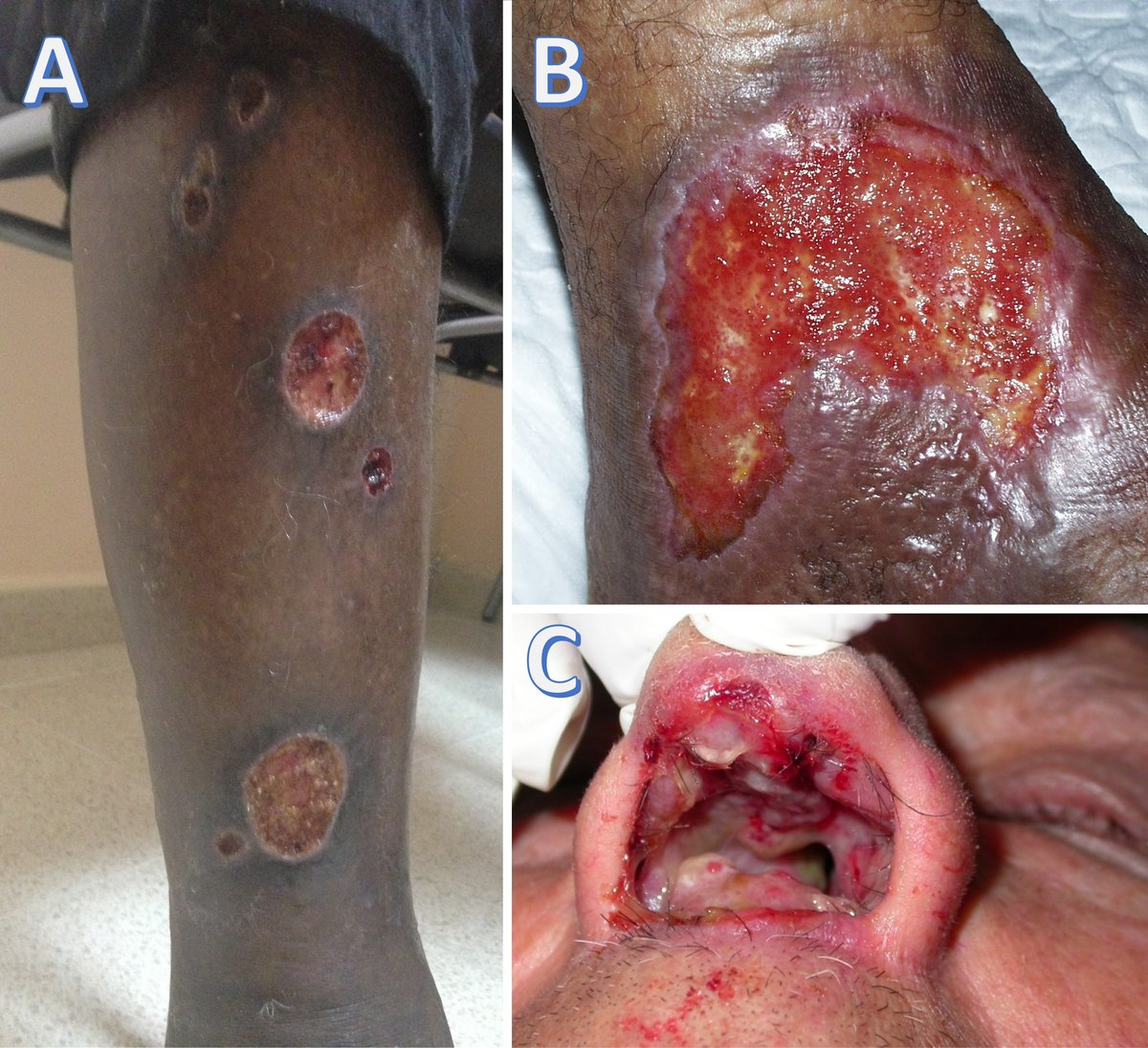
Cutaneous leishmaniasis is the most frequent form, causing ulcerated or non-ulcerated (mostly self-healing) skin lesions that mainly affect exposed areas (Figure 4a and b).
Mucocutaneous leishmaniasis occurs in South and Central America and Ethiopia. Causing large, dry, non-healing cutaneous and mucocutaneous ulcers, the nasal septum is the most common site of infection (Figure 4c). The larynx and pharynx can also be affected (Table 1).
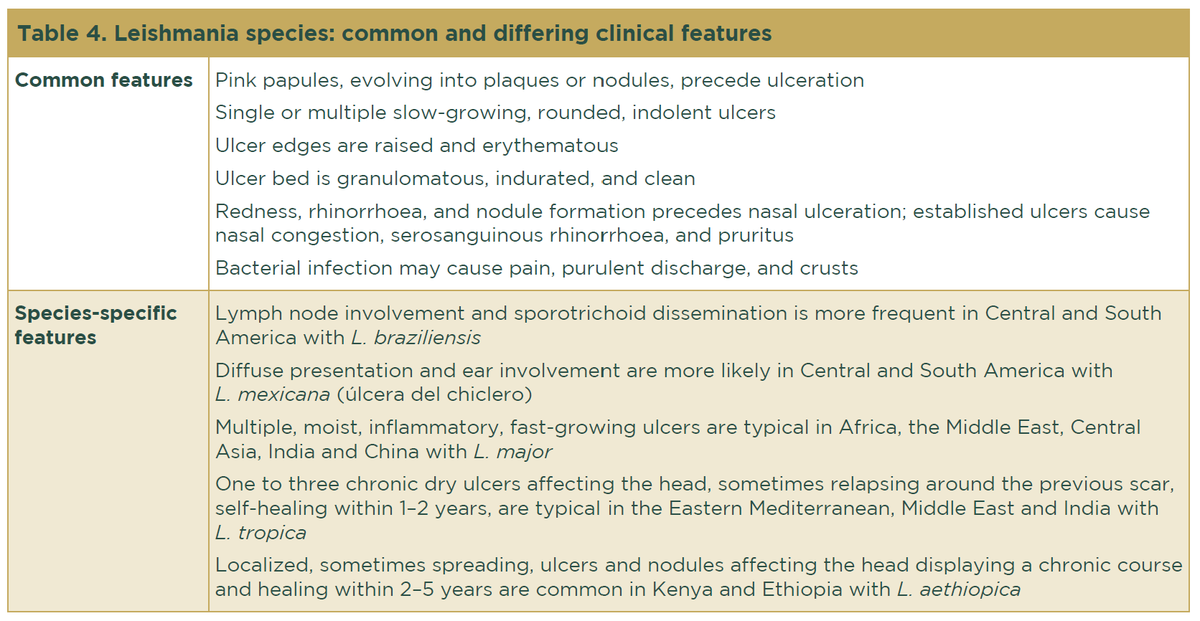
The clinical features, intensity of infection, and disease evolution vary greatly depending on the immune status of the host and the Leishmania species, the latter of which depends upon geographical area (Table 4).
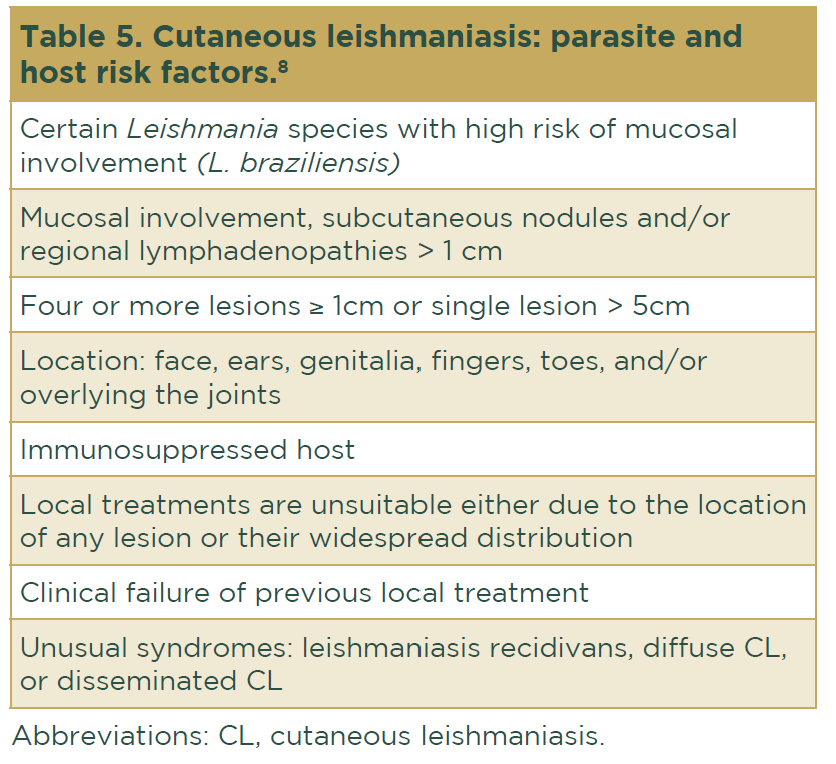
Some risk factors for CL have been reported9 (Table 5).
In contrast with VL, serological tests have little value in CL and MCL diagnosis. Direct microscopic visualisation of Leishmania amastigotes by examination of Giemsa-stained scrapings from the centre and edges of a recent ulcer is an easily accessible and very sensitive test. If available, biopsy and molecular tests are also very useful, although they require more diagnostic tools than scraping.
Treatment
For leishmaniasis, early treatment is essential. The vector, other animal reservoirs, environmental control, and eventual immunocompromised status of the patient must be addressed.
Uncomplicated cases are those considered to have no risk factors (Table 5). In this situation, a combination of local treatments is recommended according to availability:
- Intralesional antimonial;
- Cryotherapy;
- Heat thermotherapy;
- Paromomycin cream;
- Intralesional pentamidine.
Complicated cases are those with positive risk factors (Table 5). In this situation, systemic treatment is recommended. The options are as follows:
- Liposomal amphotericin B (high efficacy, but risk of nephrotoxicity);
- Systemic antimonial 20mg/kg/day IV or IM (very painful) for 10–30 days;
- Systemic antimonial plus pentoxifylline 400mg/8h for 10–20 days plus allopurinol 20mg/kg for 30 days in recurrent cases of L. tropica;
- Itraconazole 200mg/day for 42–56 days;
- Fluconazole 200–400mg/day for 42 days;
- Miltefosin 2.5mg/kg/day (maximum dose 150mg/day) divided into three oral doses for 28 days (expensive and teratogenic);
- Pentamidine 4mg/kg/48h IM or IV for 1 week (toxicity includes pancreatitis, prolongation of the ECG QT interval and hyperkalaemia);
- Chloroquine 250mg/12h and doxycycline 200mg/24h for 3 months have been proposed as alternative treatment.
References
- Noguera-Julian M, González-Beiras C, Parera M et al. Etiological characterization of the cutaneous ulcer syndrome in Papua New Guinea using shotgun metagenomics. Clin Infect Dis 2019; 68:482–9.
- Griesenauer B, González-Beiras C, Fortney KR et al. Streptococcus pyogenes is associated with idiopathic cutaneous ulcers in children on a yaws-endemic island. mBio 2021; 12:e03162-20.
- Lupi O, Madkan V, Tyring SK. Tropical dermatology: bacterial tropical diseases. J Am Acad Dermatol 2006; 54:559–78.
- Schwartzman G, Reddy SA, Berg SH et al. Cutaneous melioidosis: an updated review and primer for the dermatologist. J Am Acad Dermatol 2023; 89:1201–8.
- O’Boyle S, Barton HE, D’Aeth JC et al. National public health response to an outbreak of toxigenic Corynebacterium diphtheriae among asylum seekers in England, 2022: a descriptive epidemiological study. Lancet Public Health 2023; 8:e766–75.
- Walsh DS, Portaels F, Meyers WM. Buruli ulcer (Mycobacterium ulcerans infection). Trans R Soc Trop Med Hyg 2008; 102:969–78.
- Vandelannoote K, Buultjens AH, Porter JL et al. Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. eLife 2023; 12:e84983.
- Mitjà O, Asiedu K, Mabey D. Yaws. Lancet 2013; 381:763–73.
- Aronson N, Herwaldt BL, Libman M et al. Diagnosis and treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2016; 63:1539–57.